反应简介
傅-克酰基化反应是指一类在路易斯酸作为催化剂的条件下,芳烃与酰氯或酸酐进行酰化的反应。该反应由于羰基吸电子效应的影响,一般不会像烷基化反应生成多重酰基化产物,仅通过亲电芳香取代形成单酰化产物。[1,2]
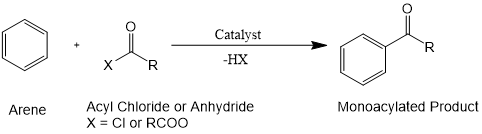
图1. 傅-克酰基化反应
反应机理
Ⅰ. 路易斯酸催化剂(AlCl3)和酰基的氯原子形成络合物,氯的解离形成酰基碳正离子。
Ⅱ. 酰基离子(RCO+)继续对芳环进行继续对芳烃进行亲电攻击。随着络合物的形成,其芳香性暂时消失。
Ⅲ. 中间态去质子化,恢复芳环的芳香性。电荷转移至氯离子形成HCl,AlCl3催化剂重新形成。[1]
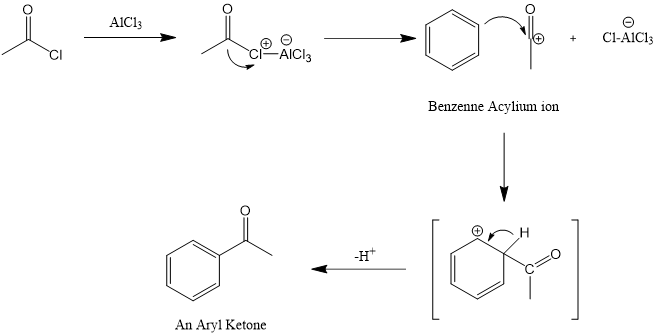
图2. AlCl3傅-克酰基化反应机理
应用
傅-克酰基化反应可应用于以下化合物的合成:
1. 二芳基乙酸衍生物[4]
2. 聚醚醚酮(PEEK)或mPEK[5]
3. 1,5-双(4-氟苯甲酰基)-2,6-二甲基萘[6]
4. 芳香酮[7]
5. 不对称芳香胺[8]
6. 环酮,例如1-四烯酮和1-茚满酮[9]
7. 2-乙酰基-6-甲氧基萘,用于合成非甾体抗炎镇痛新药萘普生及萘普酮的关键中间体[10]
研究进展
1.PVP-TfOH已经作为傅-克酰基化反应中高效且易于后处理的固体超强酸催化剂体系得以应用。在温和的反应条件下,芳烃和乙醛酸通过傅-克酰基化反应,实现了无溶剂一锅法合成二芳基乙酸衍生物。[4]
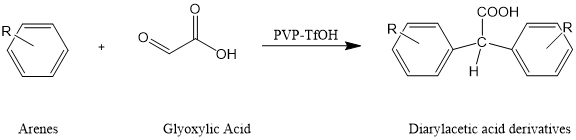
图3. 芳烃和乙醛酸通过傅-克酰基化反应,无溶剂一锅法合成二芳基乙酸衍生物
2. 一种基于咪唑的离子液体可作为催化剂,催化芳烃与乙酰氯进行傅-克酰基化反应。[11]
3. 据报道三氟甲磺酸铒是一种含有给电子基团且可用于微波辅助下芳烃的傅-克酰基化的高效催化剂。[12]
4. 虎皮楠生物碱是一类结构高度复杂多样的三萜类生物碱,可通过分子内的傅-克酰基化反应直接且快速的构建ACDE环系统。[13]
5. 酸催化的的多米诺傅-克酰基化反应,可用于高效构建台湾杉醌的6,5,6-ABC三环骨架结构。还可用于二萜(±)-甲萘醌B和(±)-二氯酮的合成。[14]
6. 通过亲电傅克酰基化缩聚反应可合成两种含1,4-萘单元的单体,新型聚(芳基酮)和聚(芳基醚酮砜)。[15]
7. 据研究,SBA-15中三苯基锡的甲苯和乙酸酐可发生傅-克酰基化反应。[16]
8. 离子液体1-异丁基-3-甲基咪唑二氢磷酸([i-BMIM]H2PO4)中的三氟甲磺酸铟在芳香族化合物与酸酐的傅-克酰基化反应中显示出强力的催化活性。[17]
9. 25,27-二烷氧基杯芳烃的傅-克酰化反应。使用酰氯和AlCl3在1,2-二氯乙烷中直接酰化脱叔丁基杯芳烃,以高产率区域选择性地提供相应的二酰基衍生物。[18]
参考文献:
1.Fox MA, Whitesell JK. 1994. Organic Chemistry. Boston: Jones and Bartlett.
2.Li JJ. 2009. Name Reactions: A Collection of Detailed Mechanisms and Synthetic Applications. Springer.
3.Sartori G, Maggi R. Advances in Friedel-Crafts Acylation Reactions. https://doi.org/10.1201/9781420067934
4.Prakash G, Paknia F, Kulkarni A, Narayanan A, Wang F, Rasul G, Mathew T, Olah GA. 2015. Taming of superacids: PVP-triflic acid as an effective solid triflic acid equivalent for Friedel?Crafts hydroxyalkylation and acylation. Journal of Fluorine Chemistry. 171102-112. https://doi.org/10.1016/j.jfluchem.2014.08.020
5.Baek J, Lyons CB, Tan L. 2004. Grafting of Vapor-Grown Carbon Nanofibers via in-Situ Polycondensation of 3-Phenoxybenzoic Acid in Poly(phosphoric acid). Macromolecules. 37(22):8278-8285. https://doi.org/10.1021/ma048964o
6.Ohno M, Takata T, Endo T. 1995. Synthesis of a novel naphthalene-based poly(arylene ether-ketone) by polycondensation of 1,5-bis(4-fluorobenzoyl)-2,6-dimethylnaphthalene with bisphenol a. J. Polym.Sci. A Polym.Chem.. 33(15):2647-2655. https://doi.org/10.1002/pola.1995.080331511
7.de Noronha RG, Fernandes AC, Romão CC. 2009. MoO2Cl2 as a novel catalyst for Friedel?Crafts acylation and sulfonylation. Tetrahedron Letters. 50(13):1407-1410. https://doi.org/10.1016/j.tetlet.2009.01.039
8.Nordlander JE, Payne MJ, Njoroge FG, Balk MA, Laikos GD, Vishwanath VM. 1984.Friedel-Crafts acylation with N-(trifluoroacetyl)-.alpha.-amino acid chlorides.Application to the preparation of .beta.-arylalkylamines and 3-substituted 1,2,3,4-tetrahydroisoquinolines. J.Org.Chem.. 49(22):4107-4111. https://doi.org/10.1021/jo00196a001
9.Tran PH, Huynh VH, Hansen PE, Chau DN, Le TN. 2015. An Efficient and Green Synthesis of 1-Indanone and 1-Tetralone via Intramolecular Friedel-Crafts Acylation Reaction. Asian Journal of Organic Chemistry. 4(5):482-486. https://doi.org/10.1002/ajoc.201402274
10.Kobayashi S, Komoto I. 2000. Remarkable Effect of Lithium Salts in Friedel?Crafts Acylation of 2-Methoxynaphthalene Catalyzed by Metal Triflates. Tetrahedron. 56(35):6463-6465. https://doi.org/10.1016/s0040-4020(00)00610-4
11.Cai M, Wang X. 2014. Activity of Imidazolium-Based Ionic Liquids as Catalysts for Friedel-Crafts Acylation of Aromatic Compounds. Asian J. Chem.. 26(18):5981-5984. https://doi.org/10.14233/ajchem.2014.16354
12.Tran PH, Hansen PE, Nguyen HT, Le TN. 2015. Erbium trifluoromethanesulfonate catalyzed Friedel?Crafts acylation using aromatic carboxylic acids as acylating agents under monomode-microwave irradiation. Tetrahedron Letters. 56(4):612-618. https://doi.org/10.1016/j.tetlet.2014.12.038
13.Wang W, Li G, Wang S, Shi Z, Cao X. 2015. Direct and Short Construction of the ACDE Ring System of Daphenylline. Chem. Asian J.. 10(2):377-382. https://doi.org/10.1002/asia.201403152
14.Tang S, Xu Y, He J, He Y, Zheng J, Pan X, She X. 2008. Application of a Domino Friedel?Crafts Acylation/Alkylation Reaction to the Formal Syntheses of (±)-Taiwaniaquinol B and (±)-Dichroanone. Org.Lett.. 10(9):1855-1858. https://doi.org/10.1021/ol800513v
15.Wen H, Wang P, Cheng S, Yan T, Cai M. 2015. Synthesis and characterization of novel organosoluble poly(aryl ether ketone)s and poly(aryl ether ketone sulfone)s containing 1,4-naphthylene units. High Performance Polymers. 27(6):705-713. https://doi.org/10.1177/0954008314557707
16.Deng Q, Qin Z, Yang Y, Song W. 2015. Synthesis, characterization of triphenyltin grafted on SBA-15 mesoporous silica and its catalytic performance for the synthesis of 4-methylacetophenone. Chinese Journal of Chemical Engineering. 23(2):384-388. https://doi.org/10.1016/j.cjche.2013.12.001
17.Tran PH, Hansen PE, Hoang HM, Chau DN, Le TN. 2015. Indium triflate in 1-isobutyl-3-methylimidazolium dihydrogen phosphate: an efficient and green catalytic system for Friedel?Crafts acylation. Tetrahedron Letters. 56(17):2187-2192. https://doi.org/10.1016/j.tetlet.2015.03.051
18.Skácel J, Budka J, Eigner V, Lhoták P. 2015. Regioselective Friedel?Crafts acylation of calix[4]arenes. Tetrahedron. 71(13):1959-1965. https://doi.org/10.1016/j.tet.2015.02.021
阿拉丁相关产品列表
产品货号
产品名称
